

|
BioDiesel Titration for Acid Value
|
||||||||||||||||||||||||||||||||
|
Materials Required
Beaker Spending money on a laboratory grade beaker for daily titrations is kind of a waste of money because you will probably break it sooner or later. Save the $10 or $15 dollar expense on a fancy lab beaker and use a regular glass food jar. Take a trip to your local grocery store and find a product that is sold in a glass jar with a volume label that says something between 150 and 250 milliliters (ml). Baby food jars are a great place to start and they are very cheap. Get a few of these. I have 10 for various purposes but you only need one. |
 |
|||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
1000 ml Graduated Cylinder
You will need a way to accurately measure out a volume of 1000 milliliters. This is done because a solution of known concentration must be created. To do this, a volume of 1000ml of distilled water is mixed with 1 gram of your catalyst. The best place I have found to obtain this piece of equipment is US Plastics.com. Follow this link to purchase. Get the poly-plastic kind so it doesn’t break when you drop it. An expensive glass cylinder probably wouldn’t last a month so don’t waste your money. |
||||||||||||||||||||||||||||||||
|
Syringes
While you’re at the grocery store, check and see if they have any syringes. If not, the local pet veterinarian or pet supply outlet will probably have something. You will need at least one syringe 2 or 3 ml in size and two syringes 10 to 20 ml in size. Plastic syringes work just fine for titrations. |
 |
|||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
Note: Do not attempt to suck up biodiesel into a plastic syringe. Biodiesel will destroy the black rubber tip of the plunger the very first time it contacts it. While titrations do not require any biodiesel sampling, quality testing does. I strongly recommend surfing Ebay for a quality two piece glass syringe in the 5ml to 10ml size range.
Note: While operating a biodiesel processor, you are almost certain to get waste vegetable oil or biodiesel on your hands and fingers. If this oil is transferred to the printed numbers and line marking on a syringe, the printed marks will dissolve and smear to become illegible. I have not found a plastic syringe that is impervious to biodiesel or waste vegetable oil but almost all glass syringes are. To solve the oily fingers problem, the best solution I have found is to wrap the outside of the syringe with normal clear packing tape. If you wrap it neatly in sections, you will never even see the tape on the syringe. You should also label each syringe for its purpose and use the syringe only for that purpose. 1) Alcohol 10ml to 30ml 2) Methanol 30ml to 60ml 3) WVO (waste vegetable oil) 2ml to 3ml 4) Titrating Solution (Titrate) 10ml to 20ml 5) Tap Water 60ml to 100ml (Optional) |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
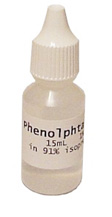
Indicating Solution
An indicating solution is a chemical that is added to your sample that will drastically change colors at a specified pH. If you are unfamiliar with the term pH, don’t worry about it at this point. You don’t need to know the exact cooking temperature of the fry pan to cook an egg. The very best indicating solution is a chemical called phenolphthalein. It is a very common chemistry supply item and can be obtained in small quantities from many laboratory supply places or biodiesel supply sites. I get mine from www.b100supply.com because it comes packaged as a pre-dissolved solution of 91% isopropyl alcohol in a little 15ml dispenser bottle that is perfect for biodiesel titrations. |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
As an alternative, you can also use a substance called Turmeric. This is common dry powder food spice or herb that is cheap and available at almost all grocery stores. Nature has seen fit to make Turmeric change its color from bright yellow to bright red at almost the perfect pH (8.5) for biodiesel titrations. While the actual color change indication that is observed is not quite as sharp as phenolphthalein, Turmeric is available almost everywhere and works good enough.
|
 |
|||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
To make a Turmeric solution, mix (by volume) one part dry Turmeric powder with ten parts of Isopropyl Alcohol of at least 90% purity. Shake the solution up and let settle until the liquid on top is transparent orange. Using the alcohol syringe, suck up the liquid on top and put it into an eye dropper bottle. Make sure you’ve cleaned the eye dropper bottle out with clean alcohol first. That small amount will last you a hundred or so titrations. You only need 5 or 6 drops each time you use it.
Note: The actual measurements of alcohol and Turmeric are not very important. You don’t even need to actually measure, just eye-ball the best estimation you can. Note: While there is nothing toxic or hazardous about Turmeric, if you get it on your fingers or clothing, it will turn them orange for a long time. Just look at the picture of the eye dropper bottle. |
||||||||||||||||||||||||||||||||
|
Isopropyl Alcohol
In order to titrate waste oil, it needs to be dissolved in alcohol. Isopropyl Alcohol is a very common substance better known as Rubbing Alcohol. It’s what a medical doctor rubs on your skin before giving you a shot. Most Isopropyl Alcohol is sold at 70% purity but we need 90%. Many major super-stores carry both but if you have a problem finding the higher purity, there is another product you can use. The product is called Iso-Heet, it comes in a red bottle and is available at most gas stations and automotive supply stores. Use only the RED bottle as shown. You will only use 10ml for each titration you do so a single bottle can last months. I usually buy two at a time. |
 |
 |
||||||||||||||||||||||||||||||
|
Catalyst Scale
When making Titration Solution, you will be required to accurately weigh a very small amount of the catalyst you choose to use. I use the Single Step Method described later in this document because my scale is capable of weighing to just .0001 grams. These kinds of scales cost thousands of dollars and while they are nice to have, they are by no means required. The best scale I have found for the relative cost is one called "Lucky 11". It costs less than $15 and does a fine job as a catalyst scale. You can find this scale at http://www.utahbiodieselsupply.com/biodieselscales.php |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
Making Titration Reference Solution
The first step to titrating vegetable oil is to make up a premixed fluid of known concentration. This is called titration solution and is used as a measuring tool to calculate the minimum amount of catalyst (lye) required to make quality fuel. If your catalyst scale is only capable of measuring to 0.1 grams, you should use the two step method. Note: The catalyst that is used to make biodiesel is hygroscopic. This means it will suck moisture right out of the air almost like magic. If you put a few flakes on a work bench, you will see the flake begin to look wet and shiny after about 60 seconds. This is the hygroscopic action taking place. If the catalyst sucks up to much moisture from the air, it will loose its potency and no longer do its job. To avoid this, you should measure out the sample as quickly as possible and screw the lid back on your supply container. Do not let the catalyst sit casually on the table while you figure out what to do next. Plan ahead so your catalyst is exposed to the air for as short of time as possible. Note: The catalyst will soak up moisture on a rainy or humid day faster than a dry day. |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
| Single Step Method Step 1 Measure out 1000ml of Distilled water using a graduated cylinder. |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
Dump this water into a plastic container that has an air tight lid. I use a 2 liter pop bottle.
|
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
Step 2
Measure out 1 gram of catalyst to an accuracy of 0.01 grams or better. |
||||||||||||||||||||||||||||||||
|
Step 3 Dump the catalyst into the distilled water, screw cap on and shake until it completely dissolves. |
||||||||||||||||||||||||||||||||
|
Two Step Method
Step 1 Measure out 1000ml of Distilled water using a graduated cylinder. Step 2 Step 3 Step 4 Step 5 Step 6 |
||||||||||||||||||||||||||||||||
|
Titrating Waste Vegetable Oil
For the purpose of these instructions, we are using a regular laboratory grade glass beaker because it is easier to photograph and display for this document. In practice, I use a baby food jar because beakers break to easy in a work shop environment. Step 1 Using a titration beaker, add 10ml of Isopropyl Alcohol (>90%) and 5 drops of Phenolphthalein or Turmeric solution. While Phenolphthalein will not change the appearance of the Isopropyl Alcohol, Turmeric will turn it bright yellow as shown. |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
Step 2
Pick up the beaker and swirl the solution while adding 1 drop at a time of titration solution until you see a slight color change that holds for 20 seconds. If the alcohol you are using is fresh the solution in the beaker should change color with just a couple drops. (Mine usually requires just 1 drop) As shown in the photo, alcohol and turmeric solution has turned a medium red color with a yellowish hue on the edges. If you are using Phenolphthalein, the solution will turn a light pink color. |
 |
|||||||||||||||||||||||||||||||
|
Note: The point at which the color change starts to occur is the point at which you stop adding titration solution. Continuing to add titration solution after the initial color change has occurred will result in the solution turning a deeper and more pronounced color. This deep color change is not what you are aiming for. Stop adding titration solution when you see the first signs of a color change that stays for at least 20 or 30 seconds.
The procedure you just performed in Step 1 and 2 is called “Blanking” the titration. It is a way to check and make sure the alcohol you are using is fresh. Alcohol goes acidic as it ages and that can throw off a titration. Once you have checked your alcohol and have determined only 1 drop is needed, you only need to check it once every week or two. If you need more than 3 or 4 drops, your alcohol is acidic and you will need to blank the titration every time or find some fresher alcohol. |
||||||||||||||||||||||||||||||||
|
Step 3
Add exactly 1ml of the oil you wish to test to the beaker. Swirl it all up and you will notice the color change back to yellow again. This time, the liquid should be slightly cloudy. This is because you just added the used acidic oil. The more stuff that was cooked in the oil, the more acidity the oil will have and usually the cloudier it will become. Note: Your alcohol solution should be at room temperature. Step 4 Suck up exactly 10ml of Titration Solution and begin adding that to the beaker a few drops at a time. It really doesn’t matter how much titration solution you suck up in the syringe, but you must pay attention to the exact amount you add to the beaker. Starting at 10ml makes that easy. |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
Once the color stays changed, record the amount, in milliliters, of Titration Solution you required. This is your Titration Number. This number tells you exactly how much extra catalyst will be required to convert the raw vegetable oil to quality fuel. This number also tells you a little bit about the quality of food at the restaurant it came from. The lower the titration number, the better the food usually is.
When you are ready to make a batch of biodiesel you will add a “Base” amount of catalyst to the Titration Number for that batch. You will then multiply this number by the number of liters in your batch. This is easy. Just follow along. If you are using Potassium Hydroxide (KOH), your base number is 7 grams. If the Titration Number was 5 then you simply add (7 grams base) + (5 Titration Number) = (12 grams Reaction). You need 12 grams of catalyst for every Liter of oil. If your batch size is 180 Liters then you multiply 12 grams per liter x 180 Liters. You will require 2160 grams (2.16KG) of catalyst for your reaction. If you are using Sodium Hydroxide (NaOH), your base number is 5 grams. If the Titration Number was 5 then you simply add (5 grams base) + (5 Titration Number) = (10 grams Reaction). You need 10 grams of catalyst for every Liter of oil. If your batch size is 180 Liters then you multiply 10 grams per liter x 180 Liters. You will require 1800 grams (1.8KG) of catalyst for your reaction. A note on catalyst purity: |
||||||||||||||||||||||||||||||||
